High Quality RNA Extraction from Murine Tissues: Balancing Preservation and Extraction Parameters for Cryogen-Free Processing
April 8, 2025Efficient RNA extractions form the foundation of success for a variety of modern transcriptomic analysis workflows. Unlike DNA, which can be extracted from a variety of esoteric samples due to its’ stability, RNA is fragile and readily degrades.1 RNase activity and the single stranded structure of RNA account for much this instability.2 This inherent obstacle necessitates researchers to often optimize their tissue RNA extractions around time consuming and cumbersome temperature regulated extraction environments. When evaluating specifically tissue RNA extractions protocols, commonly utilized RNA isolations methods often involve liquid nitrogen grinding. However, this approach is labor-intensive, low throughput, requires specialized equipment, and may not be feasible in all laboratory settings.
Bead beating is an alternative for many of these liquid nitrogen dependent tissue homogenization methods offering a rapid, scalable, and high-throughput approach suitable for a variety of sample matrices. Despite its advantages, concerns regarding heat generation during bead milling have been raised, as elevated temperatures can accelerate RNA degradation. To address this, some researchers have adopted cryogenic bead milling to mitigate thermal effects. However, this approach introduces additional complexity and cost, limiting its practicality for routine laboratory applications. Consequently, there is a need for optimized RNA extraction protocols that balance efficiency, accessibility, cost and RNA integrity.
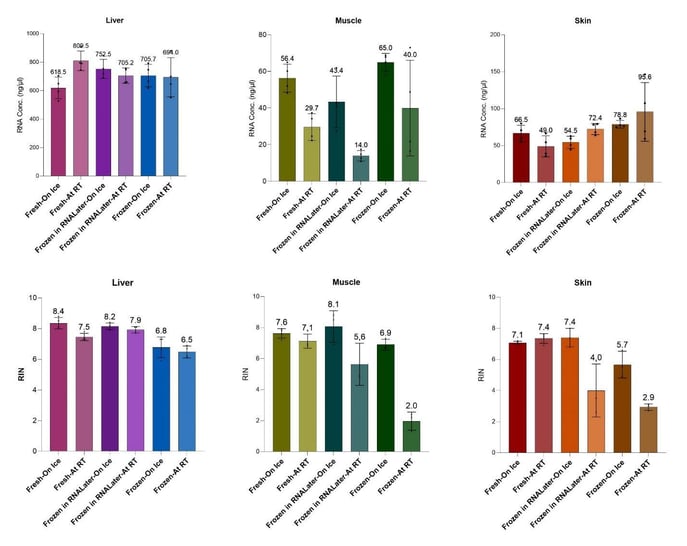
In this application note, we present an optimized RNA extraction strategy tailored for three distinct murine tissues: liver, skin, and muscle, representing a spectrum of tissue matrices and their structural complexity. Liver, a soft and readily disrupted tissue, contrasts with skin and muscle, which are more fibrous and difficult to homogenize. We demonstrate that cryogenic conditions are not essential for effective RNA extraction. Instead, by optimizing sample storage and extraction conditions, we achieve high-quality RNA yields across all tissue types. This streamlined approach provides a practical and accessible solution for researchers working with diverse and challenging samples, without compromising RNA integrity or requiring specialized cryogenic equipment.
To View the Full Application Note Please Complete the Form Below
2 Trivedi, C. B., Keuschnig, C., Larose, C., Rissi, D. V., Mourot, R., Bradley, J. A., ... & Benning, L. G. (2022). DNA/RNA preservation in glacial snow and ice samples. Frontiers in Microbiology, 13, 894893.
